A drastic shift in national policy recommends that only people 65 and older and others with certain health risks should get annual covid-19 vaccines, but at least one vaccine expert says the guidance raises as many questions as it answers.
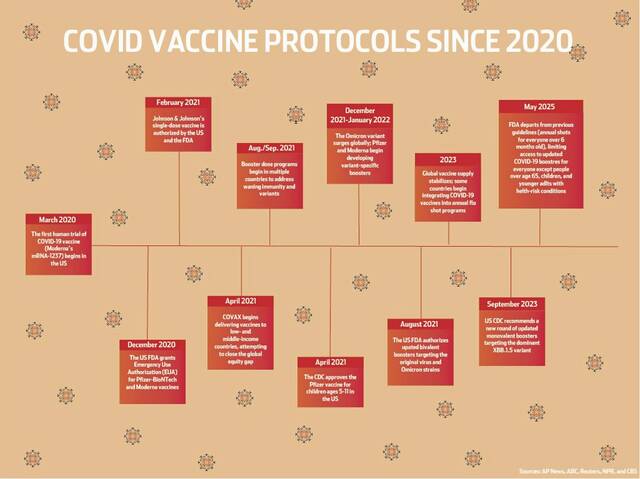
The Food and Drug Administration published its new policy Tuesday in the New England Journal of Medicine. It questions “the benefit of repeat dosing” — particularly among people who are considered to be at low risk, who may already have received multiple doses of covid-19 vaccines, or others who have contracted covid-19 more than once.
But it isn’t clear who is supposed to verify if a person meets those risk factors, said Dr. Paul Offit, 75, a vaccine expert at Children’s Hospital of Philadelphia.
“Therein lies the problem. Many people get their vaccines from a pharmacy,” he told TribLive on Wednesday. “I’m not sure how all this plays out.”
Offit plans to continue receiving the vaccine annually because he is older than 65, an age group considered at risk.
It also might mean those who want the vaccines but do not have any of the two dozen risk factors might need to pay out-of-pocket for it, Pittsburgh-based infectious disease expert Dr. Amesh Adalja said.
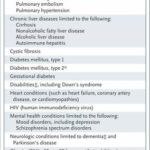
The risk factors include a host of respiratory conditions, cancer, diabetes, mental health issues and other ailments that plague many Americans.
People are encouraged to speak with their healthcare provider regarding specific questions about vaccinations, said Dr. Michael Fiorina of Butler Memorial and Clarion hospitals.
Dr. Fiorina and Dr. Carol Fox, chief medical officer of Independence Health System, said they are awaiting more guidance from the FDA and insurers in regards to those who want the vaccine but do not fit the guidelines.
A spokesperson for UPMC Health Plan, one of the region’s leading insurers echoed those comments.
“UPMC clinicians are happy to help individual patients determine which vaccines are recommended for them, based on their health status” the health plan said in a statement.
Allegheny Health Network declined to comment about the issue.
The FDA’s proposal is in line with how other countries are dealing with covid, said Adalja, who is a senior scholar at the Johns Hopkins Center for Health Security.
The one-size-fits-all approach the U.S. has taken disregards that covid is a different disease in a low-risk person than it is for someone with other health issues, he said.
“Influenza vaccines contain three different strains of influenza, whereas the current covid vaccine contains one strain,” Adalja told TribLive. “The cadence at which influenza strain changes are needed, fairly predictable compared to covid.”
Covid-19 also is vastly different from influenza, which means the way each disease’s vaccines work is different, he said.