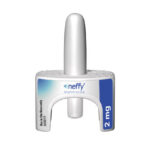
A needle-free nasal spray has been approved for serious allergic reactions to food, medications and insect stings — an alternative to the well-known EpiPen, the Washington Post reported.
The U.S. Food and Drug Administration approved the alternative 2-milligram spray, which is called Neffy, on Friday, and it’s the first needle-free treatment for allergic reactions.
Neffy will be able to treat allergic reactions that are life-threatening, or anaphylaxis, in both adult and pediatric patients who weigh over 66 pounds, according to the FDA.
Symptoms of anaphylaxis, which usually occur within minutes of exposure, include hives, swelling, itching, vomiting, difficulty breathing and loss of consciousness, the FDA said. Epinephrine is the only life-saving treatment for anaphylaxis, but it has previously only been available for patients as an injection, according to the FDA.
“Anaphylaxis is life-threatening, and some people — particularly children — may delay or avoid treatment due to fear of injections,” said Dr. Kelly Stone, associate director of the Division of Pulmonology, Allergy and Critical Care in the FDA’s Center for Drug Evaluation and Research in a release. “As a result, Neffy provides an important treatment option and addresses an unmet need.”
The new nasal spray is able to be administered as a single dose in one nostril, the Post reported. The FDA said a second dose using a new Neffy device in the same nostril “may be given if there is no improvement in symptoms or symptoms worsen.”
Richard Lowenthal, chief executive of ARS Pharmaceuticals, which is the manufacturer of Neffy, said in an interview that Neffy will help people with severe allergies live their lives normally, according to the Post.
“People don’t want to inject themselves, so they wait and hesitate,” Lowenthal said, the Post reported. Instead of worrying about having a reaction at a restaurant, he said, “they have something to use that they’re not afraid of.”
The cash price of Neffy will be $199, and it will come in a pack of two single-use devices, the Post reported. People with commercial insurance will be able to get Neffy with a $25 co-pay after applying coupons, according to Lowenthal.
The approval of Neffy was based on four studies in 175 healthy adults who didn’t have anaphylaxis, the FDA said. It was found that using the nasal spray left a comparable concentration of epinephrine in the blood as injectable products, the Post reported.
The FDA cautioned patients who may have nasal conditions, such as nasal polyps or a history of nasal surgery, to consult with their health care provider — as it could affect the absorption of Neffy.
Neffy is expected to be available in the United States within eight weeks of FDA approval, according to ARS.
The next challenge, according to the Post, will be getting Neffy to be “as ubiquitous” as the EpiPen is in schools, movie theaters, recreation venues and other common areas.