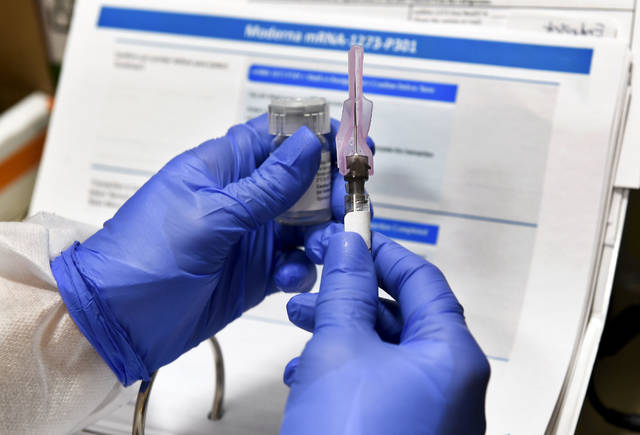
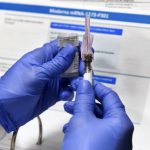
With the alarming rise of covid-19 cases in Allegheny County and throughout Pennsylvania comes the good news surrounding another vaccine.
Moderna said Monday its experimental vaccine is 94.5% effective at preventing illness, according to preliminary data from the company’s still ongoing study.
Last week Pfizer Inc. announced its own covid-19 vaccine is about 90% effective in preventing the illness.
Pittsburgh-based infectious disease and critical care physician Dr. Amesh Adalja said the Moderna vaccine, if it is approved, may have some logistical advantages over the Pfizer vaccine.
In an interview Monday with Triblive.com, Adalja, a senior scholar at the Johns Hopkins Center for Health Security, answered questions about who will determine what vaccine people receive and what options they might have, how the vaccines will be distributed and how long immunity provided by the vaccines will last.
Trib: Is one vaccine better than another?
Adalja: You could say that the Moderna vaccine, if it is approved, may be logistically easier to give to people than the Pfizer vaccine because it doesn’t require that extreme cold chain of keeping things at -70, -80 degrees centigrade and that makes it easier to develop a distribution plan. Right now we don’t have head-to-head trials on the efficacy, how well the vaccine works, or head-to-head trials on the safety of the vaccine. We’re going to have to have a menu of vaccines and there may be a time where we say certain vaccines are better for certain age groups.
But right now the way that we’re comparing them is looking at their efficacies in their placebo control trials and their storage conditions, how easy it is to give them. If you look at them that way, Moderna does seem to have a little bit of an advantage because it doesn’t need to be kept at such cold temperatures.
Q: Who will determine what vaccine people will receive and will people have an option?
A: Right now the first thing that will happen is we need to get emergency use authorization for these vaccines and we don’t have emergency use authorizations in the United States for any of these vaccines. Once that step is crossed, there’s going to be a prioritization process to see who gets the vaccine first. It’s likely to be health care workers. It’s likely to be high-risk individuals. And if there are more than one, I think they will be probably be used interchangeably. If places can do the cold storage, they can use the Pfizer vaccine. If they can’t, maybe the Moderna one will be the one that they use.
So, I think that this is going to be a challenge for Operation Warp Speed to determine how this is going to roll out if we have more than one.
Q: Will the vaccine be distributed by region? Someone might wonder ‘if the vaccine I want is available in Ohio but I live in Pennsylvania, will I be able to go across the border to get the one in Ohio?’ Or should people not be thinking that way?
A: There is no reason right now, I’m looking at Pfizer or Moderna, to prefer one or the other personally. There’s a difference in preferring it if you are a state health department, because you’ve got to figure out how to keep the vaccine cold. So, really the Moderna and Pfizer vaccines, to my knowledge, look to be interchangeable for the recipient of the vaccine. So, there’s really no reason to be going across the border to get the Moderna vaccine if your state is offering the Pfizer vaccine. It’s more about the state being able to deliver that vaccine.
Q: Do you have any concerns about the Moderna or Pfizer vaccines?
A: I have no specific concerns. Everything so far looks very good in phase I, II and III clinical trials. We’re waiting to see the full safety data from the phase III clinical trials, which I think should be forthcoming in the next couple of weeks. But I also want to look at the long-term consequences. I don’t think this should hold up any kind of vaccination program, but anytime we have a new vaccine, we do follow people for several years after they’re vaccinated to see if any long-term consequences come up.
So far everything looks very good and I’m pretty confident that these are going to be safe and effective vaccines based on everything we’ve seen thus far.
Q: Do we know yet how long immunity provided by the vaccines will last? How important is this?
A: It is an important question to answer. We don’t know that yet. The only way we’re going to know that is when we get people vaccinated and follow them over time and see how they do. What happens to their levels of antibodies? What happens to the levels of T cells that the vaccine induces? And what happens to them in their life? Do they get re-infected after a certain period of time?
This is only going to be done with what are called natural history studies. We follow vaccinated people out and measure different blood markers to see how durable that response induced by the vaccine is.
Q: Do we know yet how the vaccine will be distributed?
A: We don’t know particularly all those details yet. This is likely going to differ from state to state. It is part of Operation Warp Speed to have the states moving on these plans. So, Pennsylvania will likely have its own plan, which may be different than Ohio’s plan, and this is going to fall to the state health departments to develop a plan, working with county and city health departments across the state, to be able to try and figure out the optimal way to get this vaccine into the arms of Pennsylvanians.
Q: Who will pay for the vaccine? Will people get them for free?
A: Well, nothing is for free. So, it’s actually being paid for by taxpayers. Operation Warp Speed is a taxpayer-funded initiative. It’s not something where you’ll have an out-of-pocket cost for it, but it is something that we will pay for through our taxes.