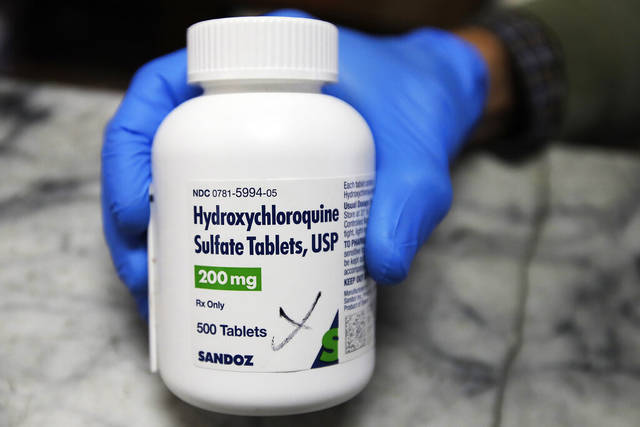
A clinical study evaluating whether hydroxychloroquine can prevent covid-19 infections in people at risk of getting the virus found that it isn’t more effective than a placebo, according to a report published Wednesday in the New England Journal of Medicine.
The conclusion of the study, which was carried out in the United States and Canada, involved more than 800 participants. All had direct exposure to a covid-19 patient, either because they lived with one, or were a health care provider or first responder.
BREAKING: Randomized trial of hydroxychloroquine w/ high risk of COVID. Other studies continue.
— Andy Slavitt @ ? (@ASlavitt) June 3, 2020
No benefit compared to a vitamin pill.
The study was the first controlled clinical trial of hydroxychloroquine – the so-called “gold standard” – and was set up to compare what occurred in people given the drug within four days of exposure and those given a placebo — or in this case, a vitamin — instead.
“I think in the setting of post-exposure prophylaxis, it doesn’t seem to work,” said Sarah Lofgren, an assistant professor at the University of Minnesota who is a co-author of the study.
The study randomized 800 participants to get either #hydroxychloroquine or placebo ("placebo-controlled") and was double-blinded (neither participants nor researchers knew what the participants received). This is the gold standard for clinical trials.https://t.co/f0ohTSKips
— Dr. Dena Grayson (@DrDenaGrayson) June 3, 2020
The results were the latest development in a highly charged medical and political issue — the efficacy of hydroxychloroquine in combating covid-19.
President Trump has repeatedly touted the anti-malarial drug as a “game changer” and recently said he took it “every day” for several days.
“The take-home message for the general public is that if you’re exposed to someone with covid-19, hydroxychloroquine is not an effective post-exposure, preventive therapy,” the lead author of the study, Dr. David R. Boulware, from the University of Minnesota, told the New York Times.
Meanwhile, after suspending the hydroxychloroquine arm of a clinical trial of experimental covid-19 drugs, the director-general of the World Health Organization said experts had reviewed the safety data and were now recommending the trial continue, the Associated Press reported.
The World Health Organization is set to resume its trial of hydroxychloroquine for potential use against the coronavirus, said WHO head Tedros Adhanom Ghebreyesus https://t.co/lt6WLiKuVx pic.twitter.com/KIaRYYX7BL
— Reuters (@Reuters) June 3, 2020
The WHO temporarily suspended its trial for the antimalarial drug last week after an analysis published in The Lancet showed coronavirus patients who took hydroxychloroquine or its related drug chloroquine were more likely to die or develop an irregular heart rhythm.