A blood pressure medication is being recalled, according to a U.S. Food and Drug Administration report, but patients prescribed the drug are advised to continue taking it until they talk to their doctor.
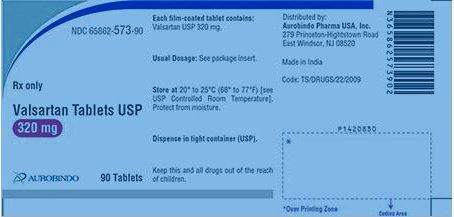
Aurobindo Pharma USA, Inc. is conducting a voluntary recall of 80 lots of Amlodipine Valsartan Tablets USP, Valsartan HCTZ Tablets USP and Valsartan Tablets USP.
The medicines are used to control blood pressure and treat heart problems.
The advisory was issued because a trace amount of the impurity NDEA, classified as a probable carcinogen, was detected in the bottled medication.
The company hasn’t received any reports of ill patients related to the drugs.
The FDA alert said patients should keep taking the medicine until they talk with their doctor because of the increased risk of stopping prescribed medication without alternative treatment.
Patients can check their medicine against a complete recalled list on the FDA website.
For more, call Aurobindo Pharma USA Inc. at 1-866-850-2876, option 2 or email pvg@aurobindousa.com.
Tawnya Panizzi is a Tribune-Review staff writer. You can contact Tawnya at 412-782-2121, ext. 2, tpanizzi@tribweb.com or via Twitter @tawnyatrib.